Bio-Photonic Research Group
Research at the Bio-Photonics Research Group (BPRG), headed by prof. Kevin Braeckmans, focuses on the development of biophotonics methods for biomedical applications and drug delivery in particular.

Prof. Dr. Kevin Braeckmans
Research at the BPRG is structured according to the following 4 themes:
Light triggered drug delivery
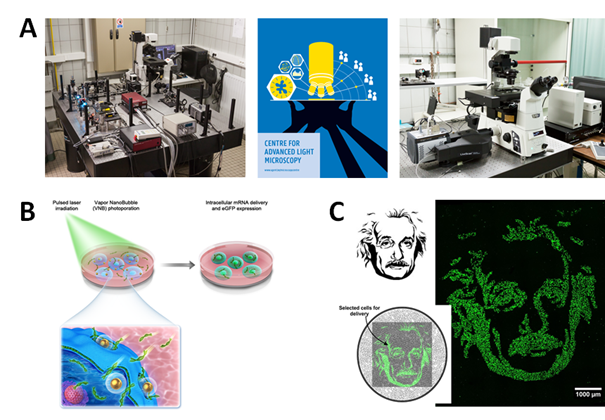
Photothermal nanoparticles are designed to be able to efficiently absorb (laser) light and convert this energy into heat or even mechanical forces. We are using this principle to enhance the delivery of effector molecules in cells, tissues and biofilms. Photothermal forces can, for instance, be used to permeabilize the outer cell membrane, allowing efficient influx of external cargo molecules. This principle is known as photoporation, which we explore to enable safe engineering of therapeutic cells for cancer therapy and regenerative medicine.
Alternatively, photothermal nanoparticles can be used as nanocarriers for biomolecules, offering the possibility of light-triggered drug release. Apart from development of bespoke optical devices and set-ups, we explore different biocompatible photothermal nanomaterials to enhance the biocompatibility of the procedure. Part of the technology is further developed in the spin-off company Trince.
- Fraire J.C., Shaabani E., Sharifiaghdam M., Rombaut M., Hinnekens C., Hua D., Ramon J., Raes L., Bolea-Fernandez E., Brans T., Vanhaecke F., Borghgraef P., Huang C., Sauvage F., Vanhaecke T., De Kock J., Xiong R.*, De Smedt S., Braeckmans K. Light-Triggered Nanobombs: Nanoscale Biolistics for Efficient Intracellular Delivery of Functional Macromolecules in Mammalian Cells. Nature Communications 13, Art nr. 1996 (2022).
- Xiong R., Hua D., Van Hoeck J., Berdecka D., Léger L., De Munter S., Fraire J., Raes L., Harizaj A., Sauvage F., Goetgeluk G., Pille M., Aalders J., Belza J., Van Acker T., Fernandez E.B., Si T., Vanhaecke F., De Vos W., Vandekerckhove B., van Hengel J., Raemdonck K., Huang C., De Smedt S.C., Braeckmans K. Photothermal nanofibers enable safe engineering of therapeutic cells. Nature Nanotechnology 16, 1281-1291 (2021).
- Harizaj A., Wels M., Raes L., Stremersch S., Goetgeluk G., Brans T., Vandekerckhove B., Sauvage F., De Smedt S.C., Lentacker I., Braeckmans K. Photoporation with biodegradable polydopamine nanosensitizers enables safe and efficient delivery of mRNA in human T cells. Advanced Functional Materials 33, Art. 2102472 (2021).
- Teirlinck E., Xiong R., Brans T., Forier K., Fraire J., Van Acker H., Matthijs N., De Rijcke R., De Smedt S.C., Coenye T., Braeckmans K. Laser-Induced Vapour Nanobubbles Improve Drug Diffusion and Efficiency in Bacterial Biofilms. Nature Communications 9, Article Number 4518 (2018).
Development of Advanced Light Microscopy methods for pharmaco-kinetic imaging
Transport of drugs and nanomaterials through biomaterials and tissues is an important issue in drug delivery. To optimize (nano)material design, methods are needed to accurately measure transport phenomena of molecules and nanoparticles at the micrometer scale. To this end we have developed tailored light microscopy methods to measure molecular and nanoparticle mobility in situ. These include biophysical models for quantitative fluorescence recovery after photobleaching (FRAP), fluorescence correlation spectroscopy (FCS) and single particle tracking (SPT). In addition we have developed optofluidic devices for improved detection and imaging of nanoparticles. Our microscopy equipment and expertise is made available through the Ghent Light Microscopy (GLiM) core facility.
- Xiong R., Vandenbroucke R.E., Broos K., Brans T., Van Wonterghem E., Libert C., Demeester J., De Smedt S.C., Braeckmans K.. Sizing nanomaterials in bio-fluids by cFRAP enables protein aggregation measurements and diagnosis of bio-barrier permeability. Nature Communications, DOI 10.1038/NCOMMS12982 (2016).
- Lorén N., Hagman J., Jonasson J.K., Deschout H., Bernin D., Cella Zanacchi F., Diaspro A., McNally J.G., Ameloot M., Smisdomh N., Nydén M., Hermansson A.M., Rudemo M., Braeckmans K. Fluorescence recovery after photobleaching in material and life sciences: putting theory into practice. Q. Rev. Biophys. 48, 323-387 (2015).
- Deschout H., Cella Zanacchi F., Mlodzianoski M., Diaspro A., Bewersdorf J., Hess S.T., Braeckmans K. Precisely and accurately localizing single emitters in fluorescence microscopy. Nature Methods 11, 253-266 (2014).
- Braeckmans K., Buyens K., Bouquet W., Vervaet C., Joye P., De Vos F., Plawinski L., Doeuvre L., Angles-Cano E., Sanders N.N., Demeester J., De Smedt S.C. Sizing nanomatter in biological fluids by fluorescence Single Particle Tracking. Nano Letters 10, 4435-4442 (2010).
Investigating biological barriers to nanomedicines
In drug delivery, intensive research is being carried out to develop ‘intelligent’ nanocarriers that are capable of efficiently delivering biopharmaceuticals to target cells. Obtaining a better insight in the ability of nanomedicines in crossing biological barriers that are of relevance to the delivery process is required to achieve efficient optimization of their structure and composition. Therefore, we are investigating the dynamic interaction of nanomedicines with various biological tissues. Special attention has gone to determining the colloidal stability of nanomedicine formulations in undiluted biological fluids, such as blood. Other studies have focused on characterizing nanomedicine mobility in extracellular tissues like cystic fibrosis mucus or vitreous humour. Also cellular uptake and intracellular processing of nanomedicines has been investigated in great detail. By providing a better insight into the stability and transport of nanoparticles during the various phases of the delivery process through the use of advanced microscopy techniques, it is our aim to enable a more efficient and rational development of improved carrier materials for the delivery of nucleic acids.
- Vermeulen L., Brans T., Samal S., Dubruel P., Demeester Jo., De Smedt S., Remaut K., Braeckmans K. Endosomal Size and Membrane Leakiness Influence Proton Sponge-Based Rupture of Endosomal Vesicles. ACS Nano 12, 2332-2345 (2018).
- Forier K., Messiaen A.S., Raemdonck K., Nelis H., De Smedt S.C., Demeester J., Coenye T., Braeckmans K. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J. Control. Release 195, 21-28 (2014).
- Vercauteren D., Deschout H., Remaut K., Engbersen J.F.J., Jones A.T., Demeester J., De Smedt S.C., Braeckmans K. Dynamic Colocalization Microscopy to Characterize Intracellular Trafficking of Nanomedicines. ACS Nano 5, 7874-7884 (2011).
- Braeckmans K., Buyens K., Bouquet W., Vervaet C., Joye P., De Vos F., Plawinski L., Doeuvre L., Angles-Cano E., Sanders N.N., Demeester J., De Smedt S.C. Sizing nanomatter in biological fluids by fluorescence Single Particle Tracking. Nano Letters 10, 4435-4442 (2010).
Biomedical diagnostics
Optical methods can play an important role in biomedical diagnostics. For instance, cell-derived vesicles, including exosomes, are receiving a lot of attention of late as potential diagnostic markers for various diseases, such as cancer. We have been working on optical detection and characterization methods of extracellular vesicles, including by SERS fingerprinting. In addition, in collaboration with other groups we are working on projects where in vivo optical imaging and detection of cells is important, for instance in the context of cancer immunotherapy.
- Fraire J.C., Stremersch S., Bouckaert D., Monteyne T., De Beer T., Wuytens P., De Rycke R., Skirtach A.G., Raemdonck K., De Smedt S.C., Braeckmans K. Improved label-free identification of individual Exosome-Like Vesicles with Au@Ag Nanoparticles as SERS Substrate. ACS Applied Materials and Interfaces 11, 39424-39435 (2019).
- Xiong R., Joris F., Liang S., De Rycke R., Lippens S., Demeester J., Skirtach A.G., Raemdonck K., Himmelreich U., De Smedt S., Braeckmans K. Cytosolic delivery of nano-labels prevents their asymmetric inheritance and enables extended quantitative in vivo cell imaging. Nano Letters 16, 5975-5986 (2016).
- Stremersch S., Marro M., Pinchasik B.-E., Baatsen P., Hendrix A., De Smedt S.C., Loza-Alvarez P., Skirtach A.G., Raemdonck K., Braeckmans K. Identification of Individual Exosome-Like Vesicles by Surface Enhanced Raman Spectroscopy. Small 12, 3292-3301 (2016).
- Deschout H., Raemdonck K., Stremersch S., Maoddi P., Mernier G., Renaud P., Jiguet S., Hendrix A., Bracke M., Van den Broecke R., Röding M., Rudemo M., Demeester J., De Smedt S.C., Strubbe F., Neyts K., Braeckmans K. On-chip light sheet illumination enables diagnostic size and concentration measurements of membrane vesicles in biofluids. Nanoscale 6, 1741-1747 (2014).
